Ammonium nitrate
| Ammonium nitrate | |
|---|---|
 |
|
 |
|
|
Ammonium nitrate
|
|
| Identifiers | |
| CAS number | 6484-52-2 |
| UN number | 0222 – with > 0.2% combustible substances 1942 – with <= 0.2% combustible substances 2067 – fertilizers 2426 – liquid |
| RTECS number | BR9050000 |
| Properties | |
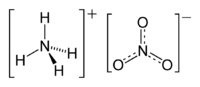
| Molecular formula | (NH4)(NO3) |
| Molar mass | 80.043 g/mol |
| Appearance | white solid |
| Density | 1.725 g/cm3 (20 °C) |
| Melting point |
169.6 °C |
| Boiling point |
approx. 210 °C decomp. |
| Solubility in water | 118 g/100 ml (0 °C) 150 g/100 ml (20 °C) 297 g/100 ml (40 °C) 410 g/100 ml (60 °C) 576 g/100 ml (80 °C) 1024 g/100 ml (100 °C) [1] |
| Structure | |
| Crystal structure | trigonal |
| Explosive data | |
| Shock sensitivity | very low |
| Friction sensitivity | very low |
| Explosive velocity | 5270 m/s |
| Hazards | |
| MSDS | ICSC 0216 |
| EU Index | not listed |
| Main hazards | Explosive |
| NFPA 704 |
 0
2
3
OX
|
| LD50 | 2085–5300 mg/kg (oral in rats, mice)[2] |
| Related compounds | |
| Other anions | Ammonium nitrite |
| Other cations | Sodium nitrate Potassium nitrate Hydroxylammonium nitrate |
| Related compounds | Ammonium perchlorate |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
The chemical compound ammonium nitrate, the nitrate of ammonia with the chemical formula NH4NO3, is a white crystalline solid at room temperature and standard pressure. It is commonly used in agriculture as a high-nitrogen fertilizer, and it has also been used as an oxidizing agent in explosives, including improvised explosive devices. It is the main component of ANFO, a very popular explosive.
Ammonium nitrate is used in instant cold packs, as hydrating the salt is an endothermic process.
Contents |
Production
The processes involved in the production of ammonium nitrate in industry, although chemically simple, are technologically challenging. The acid-base reaction of ammonia with nitric acid gives a solution of ammonium nitrate:[3]
- HNO3(aq) + NH3(g) → NH4NO3(aq)
For industrial production, this is done using anhydrous ammonia gas and concentrated nitric acid. This reaction is violent and very exothermic. After the solution is formed, typically at about 83% concentration, the excess water is evaporated to an ammonium nitrate (AN) content of 95% to 99.9% concentration (AN melt), depending on grade. The AN melt is then made into "prills" or small beads in a spray tower, or into granules by spraying and tumbling in a rotating drum. The prills or granules may be further dried, cooled, and then coated to prevent caking. These prills or granules are the typical AN products in commerce.
The Haber process combines nitrogen and hydrogen to produce ammonia, part of which can be oxidized to nitric acid and combined with the remaining ammonia to produce the nitrate. Another production method is used in the so-called Odda process.
Ammonium nitrate is also manufactured by amateur explosive enthusiasts by metathesis reactions:
- (NH4)2SO4 + 2 NaNO3 → Na2SO4 + 2 NH4NO3
- Ca(NO3)2 + (NH4)2SO4 → 2 NH4NO3 + CaSO4
Sodium sulfate is removed by lowering the temperature of the mixture. Since sodium sulfate is much less water-soluble than ammonium nitrate, it precipitates, and may be filtered off. For the reaction with calcium nitrate, the calcium sulfate generated is quite insoluble, even at room temperature.
Crystalline phases
Transformations of the crystal states due to changing conditions (temperature, pressure) affect the physical properties of ammonium nitrate. The following crystalline states have been identified:
| System | Temperature (°C) | State | Volume Change (%) |
|---|---|---|---|
| - | >169.6 | liquid | - |
| I | 169.6 to 125.2 | cubic | +2.1 |
| II | 125.2 to 84.2 | tetragonal | -1.3 |
| III | 84.2 to 32.3 | α-rhombic | +3.6 |
| IV | 32.3 to −16.8 | β-rhombic | −2.9 |
| V | −16.8 | tetragonal | - |
The type V crystal is a quasi-cubic form which is related to caesium chloride, the nitrogens of the nitrates and the ammoniums are at the sites in a cubic array where Cs and Cl would be in the CsCl lattice. See C.S. Choi and H.J. Prask, Acta Crystallographica B, 1983, 39, 414-420.
Disasters
Ammonium nitrate decomposes into gases including oxygen when heated (non-explosive reaction); however, ammonium nitrate can be induced to decompose explosively by detonation. Large stockpiles of the material can be a major fire risk due to their supporting oxidation, and may also detonate, as happened in the Texas City disaster of 1947, which led to major changes in the regulations for storage and handling.
There are two major classes of incidents resulting in explosions:
- In the first case, the explosion happens by the mechanism of shock-to-detonation transition. The initiation happens by an explosive charge going off in the mass, by the detonation of a shell thrown into the mass, or by detonation of an explosive mixture in contact with the mass. The examples are Kriewald, Morgan (present-day Sayreville, New Jersey) Oppau, Tessenderlo and Traskwood.
- In the second case, the explosion results from a fire that spreads into the ammonium nitrate itself (Texas City, Brest, Oakdale), or from a mixture of ammonium nitrate with a combustible material during the fire (Repauno, Cherokee, Nadadores). The fire must be confined at least to a degree for successful transition from a fire to an explosion (a phenomenon known as "deflagration-to-detonation transition", or DDT). Pure, compact AN is stable and very difficult to ignite, and there are numerous cases when even impure AN did not explode in a fire.
Ammonium-nitrate-based explosives were used in the Oklahoma City bombing.
Ammonium nitrate decomposes in temperatures normally well above 200°C. However the presence of impurities (organic and/or inorganic) will often reduce the temperature point when heat is being generated. Once the AN has started to decompose, then a runaway reaction will normally occur as the heat of decomposition is very large. AN evolves so much heat that this runaway reaction is normally impossible to stop. This is a well-known hazard with some types of N-P-K Fertilizers, and it is responsible for the loss of several cargo ships.
In November 2009, a ban on ammonium sulfate, ammonium nitrate, and calcium ammonium nitrate fertilizers was imposed in the Malakand Division—comprising the Dir, Swat, Chitral, and Malakand districts of the North West Frontier Province (NWFP) of Pakistan—by the NWFP government, following reports that those chemicals were used by militants to make explosives. In January 2010, these substances were also banned in Afghanistan for the same reason.
References
- ↑ Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0070494398
- ↑ Martel, B.; Cassidy, K. (2004). Chemical Risk Analysis: A Practical Handbook. Butterworth–Heinemann. pp. 362. ISBN 1903996651.
- ↑ http://www.google.com/patents/pdf/Process_of_producing_concentrated_soluti.pdf?id=XronAAAAEBAJ&output=pdf&sig=ACfU3U0iYFRDUxltKLaVind-3wwP_JYPxg
- Properties: UNIDO and International Fertilizer Development Center (1998), Fertilizer Manual, Kluwer Academic Publishers, ISBN 0-7923-5032-4.
External links
- International Chemical Safety Card 0216
- "Storing and Handling Ammonium Nitrate", UK Health and Safety Executive publication INDG230 (1986)